Achieving flawless skin as part of the desire to be perceived as ‘beautiful’ is a common sentiment shared by many cultures [1]. Of the many treatment options and products available on the market, the most common chemical agent to achieve this is hydroquinone (HQ), a topical bleaching agent used in the treatment of hyperpigmentation or dyschromias.
HQ concentrations vary from 2% (OTC) to 4-15% (Rx) and can be used independently as a topical cream or in combination with other non-hydroquinone agents such as arbutin, kojic acid, azelaic acid, vitamin C and tretinoin [2]. HQ is generally well tolerated; however, some side-effects have been documented, including erythema, mild irritant contact dermatitis, dryness, irritation and pruritis [3]. Use of this medication requires sunscreen protection post-application and careful monitoring of frequency and duration to avoid the development of exogenous ochronosis (EO). EO, a rare but serious complication of long-term, high concentration HQ use, is a localised and paradoxical cutaneous disorder characterised by diffuse, symmetrical, asymptomatic hyperpigmentation over sun-exposed skin first described in 1975 in a group of South African patients [4,5].
We present a case involving a 61-year-old female of Venezuelan decent, with olive skin tone, Fitzpatrick skin type IV, who was diagnosed with EO. Included in her 10+ year skin care regimen was HQ 4% which a plastic surgeon suggested to help achieve a more even complexion.
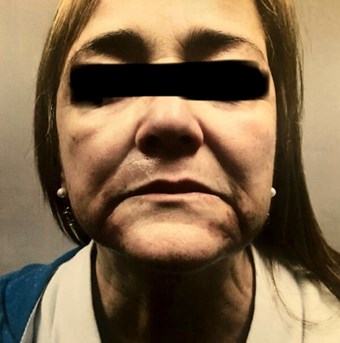


Figure 1 (above): July 2018. First consultation for
Q-Switched exogenous ochronosis treatment.
Case description
The patient was a 61-year-old female with Fitzpatrick skin type IV who presented with tan to dark-brown ill-defined bilateral hyperpigmented patches on the chin and mild to moderate hyperpigmentation on the upper lip and cheek that became worrisome two years previously. She had unremarkable dermatologic and past medical histories – except for hay fever allergic symptoms and hypertension – and denied any family history of melanoma or melasma. There was no history of pigment changes in any other areas of the body along with no systemic / extra-articular complaints.
The patient provided the following timetable of events:
2005: Several small sun marks that she attributed to high amounts of exposure in her youth. When exposed, always tans, never burns.
→ Plastic surgeon gave a product line which included retinol, HQ 4%, benzo-
peroxide, among other ingredients.
2006: Resolution of marks.
→ Patient states that she received no formal patient education on the products given outside of prescribed treatment plan.
→ To prevent any re-occurring marks, patient continues once daily for the next 11 years without sunscreen on applied areas.
2006-2016: Patient states “brighter” complexion, appeared more youthful, and felt more confident about her appearance.
2017: Noticing larger hyperpigmented patches that looked different than in first 2005 occurrence.
→ Consulted her general practitioner physician, diagnosed with mild melasma, told to continue using HQ but to increase frequency to twice daily.
→ Over the next year, the hyperpigmentation worsened.
Spring 2018: Referred to a dermatologist when undergoing an un-related laser treatment with an aesthetician as the clinician thought the patches just looked “suspicious.”
→ Right medial buccal check biopsy (0.2 x 0.2 x 0.2cm punch) confirmed exogenous ochronosis.
→ Dermatopathology microscopic exam results: within the dermis, there are small round to banana shaped yellow to somewhat orange deposits. Given the clinical impressions, these changes are consistent with ochronosis. Multiple deeper sections were performed.
→ After having discussions with the dermatologist about diagnosis and difficulty of treatment options / success rates and doing her own research, she was referred to our current dermatology practice for a consultation (Figure 1).
Summer 2018: Patient was referred to current practice and began treatment as follows.
→ Treatment: Q-Switched Laser (755nm at 1Hz).
Autumn 2018: The patient has undergone four treatment sessions since July 2018 with some self-assessed improvements, although very minimal.
Discussion
Ochronosis presents in two forms:
- Endogenous (alkaptonuria) – an autosomal recessive metabolic disorder in which homogentisic acid oxidase is deficient leading to increased urine homogentisic acid, a HQ metabolite of tyrosine, urine darkening after prolonged air exposure, blue-black pigment in collagen-containing structures, and predominant extra-articular involvement in the form of ocular symptoms, dyschromia, cardiac and genitourinary system involvement.
- Exogenous – a paradoxical hyperpigmentation adverse side-effect from skin lightening agents containing HQ without the presence of secondary systemic symptoms [4-8]. HQ inhibits enzymatic conversions of tyrosine to DOPA (dihydroxyphenylalanine) which decreases the number of melanocytes and melanin transfer leading to lighter skin [3].
The exact pathogenesis of EO is not clear; however, the most accepted theory to date is that the hyperpigmentation is due to the local competitive inhibition of HQ’s enzyme homogentisic oxidase, which leads to the local accumulation of homogentisic acid leading to its metabolic products polymerising to form the classic ‘ochre’ deposits [8]. EO is histologically defined by the yellow-brown, curvilinear, ‘banana-shaped’ ochre dermal deposits [5,7[. In EO, the severe form will present as blue-black skin [4,5,7].
EO is more common in the darker Fitzpatrick skin types IV, V, VI. However, more cases involving fair-skinned people, like Europeans and Hispanics, and with HQ 2% being used for just six months are also being reported [6,9-11]. However, one must keep in mind that the increase in cases with lower concentrations may be due to the wider availability of lower concentration products, so it is unclear how risky high versus low-concentrations are with respect to developing EO [12]. Although the actual incidence rate is unknown, 789 cases of EO have been identified worldwide with only 22 originating from the United States; however of these 789 cases, 652 fail to specify the percentage of HQ used [7,9,12]. Once thought to be a rarity in the United States, dermatologists are finding that EO more frequently presents on a spectrum than with the extremes described in many dermatological texts and can easily be misdiagnosed [5] – resulting in more HQ use. In fact, other dyschromias, like melasma, can become darker as EO develops within it because of HQ used as the mainstay of treatment [13].
The US incidence rate of EO was determined to be 37 cases from 1983 to 2014 [7]. However, the US Food and Drug Administration (FDA) Department of Health and Human Services 1998 survey completed by 2080 US dermatologists reported 512 suspected cases with 130 confirmed by pathology, demonstrating that the actual prevalence of EO in the US appears to be underreported [7]. These statistics are both interesting and alarming when discussed alongside the 2006 FDA statement release proposing a ban on over-the-counter HQ with additional modifications for the continued use of prescription HQ if not also banned. In the context of HQ-containing products and the possible side-effect of EO, we must also emphasise that the use of HQ is not all negative, when used properly. For example, one could actively use HQ with sunscreen for two weeks followed by two weeks of non-use or replacement with non-tyrosinase agents like kojic acid and tretinoin with sunscreen for three months and then follow-up with a board-certified dermatologist for re-assessment and modification of the treatment plan. This partnership, along with patient education, reduces the risk of excessive, unsupervised use which is the primary risk factor for developing EO, and not the compound HQ itself [12].
Treatment for EO is difficult and early diagnosis is important. The use of non-invasive dermoscopy and skin biopsy has been shown to diagnose EO reliably, as the differential diagnosis of hyperpigmented macules can be extensive, including bilateral nevus of Ota, drug-induced (ex: minocycline, methotrexate, amiodarone), post-inflammatory hyperpigmentation, and dermatosis papulosa nigra [6,14]. Several treatment options are available with variable outcomes. The first step is to stop using HQ and / or any HQ-containing products! Due to the difficulty in treating EO, prevention is imperative along with early detection, close medical monitoring, and sun protection to help improve outcomes. Chemical peels with glycolic acid, cryotherapy, dermabrasion, and the Q-switch Nd Yag 1064 / Alexandrite / Ruby 755 lasers have been shown to improve EO-induced hyperpigmentation [6,8]. However, care must be taken as these same modalities have been shown to inadvertently cause inflammation and irritation that results in furthering the unwanted hypermelanosis [13-15].
Further research and / or documented case reports originating from the United States are needed to help visually demonstrate the ongoing phenomena of EO to the medical community and public. Furthermore, this would make patient education and social awareness more feasible since generally, the patterns of people’s decision-making tend to be driven by their own past experiences and / or hearing from other people [16]. With that said, it is also important to recognise that the consistency of a person’s behavior over time is the result of personality and motivational factors that are common to the situations in which the behaviour occurs [16]. Traits and habits are unique to that person and environment. The demographics of the US are changing and heading towards a more ethnically diverse population. In 2012, the US Census Bureau reported that Asian and Hispanic populations will double by 2060 and that African-born people, where the highest rates of EO have been reported, will double approximately every 10 years [7].
What does this mean for dermatologists and patients, especially patients of colour? We must first recognise and accept that EO is no longer an “African or Asian problem”; secondly, we must understand the importance of partnering and utilising the knowledge and expertise of board-certified dermatologists who can provide guidance and / or the appropriate referrals; and finally, we must be mindful that increased accessibility of HQ-containing products on the internet, black market, and even other clinicians not board-certified in dermatology all pose the risk of inappropriate use.
Conclusion
HQ’s paradoxical effect of EO is an important adverse reaction and is the result of an unintended but vicious cycle that needs recognised by clinicians and consumers. With standards of beauty shaping cultures worldwide and a billion-dollar cosmetic industry capitalising on our beauty-obsessed culture, HQ-induced hyperpigmentation has the potential to cause increased psychological distress, decreases in social functioning, lower workplace productivity, and lower self-esteem [2]. EO’s high likelihood of being misdiagnosed, leading to continued HQ use and an unknowing perpetuation of the condition, makes early detection and cessation of all HQ-containing products essential for positive patient outcomes.
As the demographic in the United States continues to shift to towards being a multi-ethnic nation, while the laxity and accessibility of HQ-containing products, both prescription and over-the-counter, continues to rise, is it imperative that adequate patient education on HQ and EO be addressed both early on with a board-certified dermatologist and with more awareness as a society.
References
1. Maymone MBC, Neamah HH, Secemsky EA, et al. The most beautiful people: evolving standards of beauty. JAMA Dermatol 2017;153(12):1327-9.
2. Kang SJ, Davis SA, Feldman SR, McMichael AJ. Dyschromia in skin of color. J Drugs Dermatol 2014;13(4):401-6.
3. Hydroquinone. American Osteopathic College of Dermatology.
https://www.aocd.org/page/
Hydroquinone
[accessed 11 November 20020.
4. Gandhi V, Verma P, Naik G. Exogenous ochronosis after prolonged use of topical 2% Hydroquinone in a 50-year-old Indian female. Indian J Dermatol 2012;57(5):394-5.
5. Tan SK. Exogenous ochronosis-a diagnostic challenge. J Cosmetic Dermatol 2010;9:313-17.
6. Maxfield L, Gaston DA. Exogenous ochronosis with use of low potency hydroquinone in a Caucasian patient. J Dermatol Res Ther 2015;1(1):1-2.
7. Simmons BJ, Griffith RD, Bray FN, et al. Exogenous ochronosis: a comprehensive review of the diagnosis, epidemiology, causes, and treatments. Am J Clin Dermatol 2015;16:205-12.
8. Bhattar PA, Zawar VP, Godse KV, et al. Exogenous Ochronosis. Indian J Dermatol 2015;60(6):537-42.
9. Romero SA, Mariano AVdO, Francesconi VA, et al. Use of dermoscopy for diagnosis of exogenous ochronosis. An Bras Dermatol 2011;86:31-4.
10. Martins VMR, Portela NdC, Gonvalves LMS, et al. Exogenous ochronosis: a case report and literature review. An Bras Dermatol 2012;87(4):633-6.
11. Zawar VP, Mhaskar ST. Exogenous ochronosis following hydroquinone for melasma. J Cosmetic Dermtol 2004;3:234-6.
12. Levitt J. The safety of hydroquinone: a dermatologist’s response to the 2006 Federal Register. J Am Acad Dermatol 207;57(5):854-72.
13. Stratigos AJ, Katsamas. Optimal management of recalcitrant disorders of hyperpigmentation in dark-skinned patients. Am J Clin Dermatol 2004;5(3):161-8.
14. Taylor CR, Anderson RR. Ineffective treatment of refractory melasma and postinflammatory hyperpigmentation by Q‐switched ruby laser. J Dermatol Surg 1994;20(9):592-7.
15. Gerber PA, Kukova G, Bolke E, et al. Severe hyperpigmentation and scarring following glycolic acid peel treatment in combination with low-dose isotretinoin. Euro J Med 2014;19(60):1-4.
16. Albarracin D, Wyer RD. The cognitive impact of past behavior: influences on beliefs, attitudes, and future behavioral decisions. J Pers Soc Psychol 2000;79(1):5–22.
Declaration of competing interest: None declared.
Acknowledgement
We would like to thank the patient for allowing her case to be presented for educational purposes.
COMMENTS ARE WELCOME